Georgie Davies1 , and Rodrigo De Marco1§
, and Rodrigo De Marco1§
1Liverpool John Moores University
§Correspondence to: Rodrigo De Marco (R.J.DeMarco@ljmu.ac.uk)
Abstract
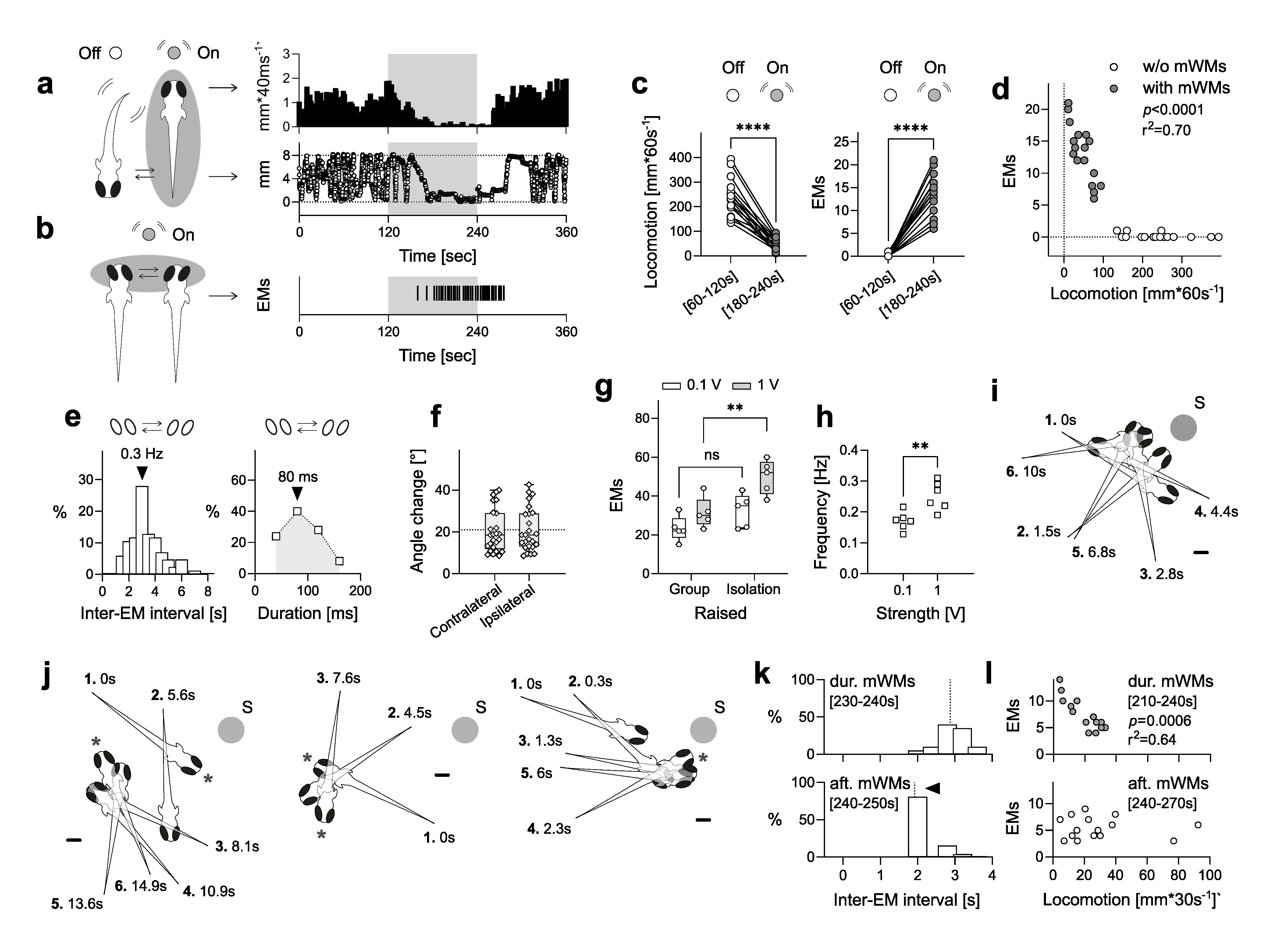
Description
Methods
Acknowledgements
Funding
Author Contributions
- Georgie Davies: Investigation, Methodology, Data curation, Visualization
- Rodrigo De Marco: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Formal analysis, Supervision, Visualization, Resources, Writing - original draft, Writing - review & editing
Reviewed By
Chintan Trivedi
Nomenclature Validated By
Anonymous
History
- Received: 9/11/2024
- Revision Received: 10/4/2024
- Accepted: 10/8/2024
- Published Online: 10/9/2024
- Indexed: 10/23/2024
Copyright
© 2024 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation
PubMed Central: 11499938
PubMed: 39450186
microPublication Biology:ISSN: 2578-9430

