Valeria Faria1*, Robby Maxwell2*, Sean Kinney2*, and Amber Hale1§*
1Department of Biology, McNeese State University
2Louisiana Department of Wildlife and Fisheries
§Correspondence to: Amber Hale (ahale@mcneese.edu)
* Authors have equal contribution
Abstract
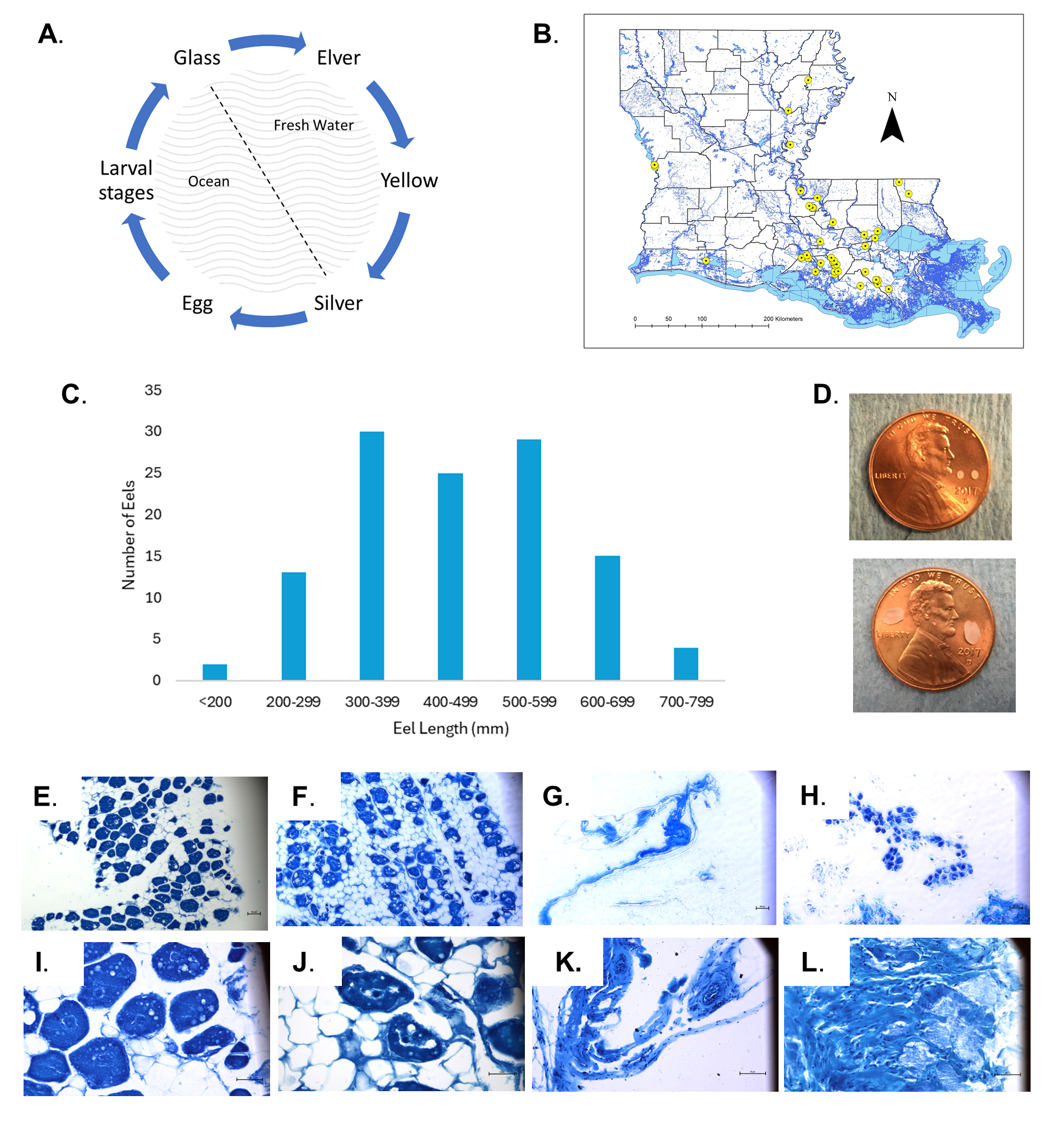
Description
Methods
Funding
Author Contributions
- Valeria Faria: Data curation, Writing - original draft
- Robby Maxwell: Conceptualization, Data curation, Writing - review & editing
- Sean Kinney: Data curation, Conceptualization, Writing - review & editing
- Amber Hale: Writing - original draft, Project administration, Funding acquisition, Data curation, Conceptualization
Reviewed By
Anonymous
History
- Received: 6/10/2024
- Revision Received: 7/10/2024
- Accepted: 7/11/2024
- Published Online: 7/12/2024
- Indexed: 7/26/2024
Copyright
© 2024 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation
PubMed Central: 11282433
PubMed: 39071172
microPublication Biology:ISSN: 2578-9430

